Steering, maintaining and promoting the adoption of Orphacodes across MS
DESCRIPTION
The proposition carried in this WP is based on the CEGRD’s “Recommendation on Ways to Improve Codification for Rare Diseases in Health Information Systems”. In order to enable countries to implement coding of rare diseases in a standardized and interoperable way the work package aims at developing a toolset to assist member states in implementing the Orphacodes in their health system. A steering group of MS will be set up to learn from local experiences already in place and better define the required steps and strategy of such a generalization of codification of RD at EU level. The definition of common guidelines addressing the issues of both quality of codification and coherence of exploitation at the European level is a major ambition of this WP. In a second step the development of a European file holding all necessary Orphacodes to be used for implementation in countries will be developed. It will be tested together with the guidelines against already existing systems and fine-tuned according to the test results. This WP is in close cross-talk with WP4, for it is necessary that Orphanet continues its work of keeping the central resource up to date, multi-lingual and interoperable with other resources in use by MS in their HIS. This WP does not address local implementation of the Orpha coding. It is meant to provide guidance and common standards in order to make sure data will be exploitable and comparable at EU level.
GOALS
- Define the common objectives for coding RD in MS, the common level of granularity to be used and guide the implementation
- Define a codification resource aimed at having consistency across MS coding for RD
- Tune the codification resource after having tested it in a subset of coding groups through pre-existing tools.
Implementation of rare disease patient coding across member states
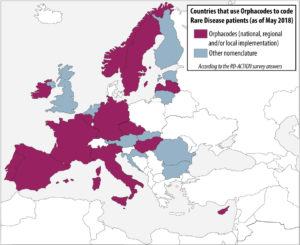
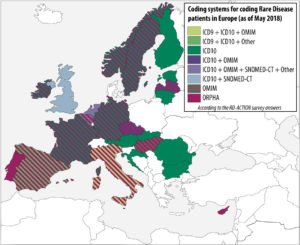
In order to analyze the level of implementation of rare disease patient coding across european member states, a survey was conducted by the co-leaders of the RD-Action Work Package 5. The results take into account the responses of 21 member states. Even if there is only a small number of countries who have already implemented strategies to produce statistics on rare diseases at national level, many have identified it as a priority. The survey indicates that rare disease patients are mostly coded in health information systems by using general coding system for morbidity/mortality, namely ICD-10. However, orphacodes emerge as the main coding system dedicated to rare diseases in both inpatient and outpatient clinics. More than 50% of the countries who answered use Orphacodes to code rare disease patients regardless of the level of implementation (national, regional or local) and 70% are in the process or already have implemented the RD-Action guidelines for orphacode implementation
Help us to complete this overview of the implementation of rare disease patient coding across member states! If you wish to contribute or have a comment about this map, please contact rare-diseases@dimdi.de
The final Codification (WorkPackage5) workshop was held in Venice, 20-21 June. The Workshop set the scene of RD coding situation in Europe and focused on the WP5 legacy and the future perspectives of Orphacodes’ use. Starting from the resources developed during the RD-action, the issue of RD coding was tackled from the perspectives of all the relevant stakeholders involved: WHO, Orphanet, JRC, ERNs. Examples of orphacodes’ use at regional/national level (Generalitat Valenciana-Spain, Portugal, Ireland) have been presented as well. The agenda (here) and the presentations (here) are available.